Relapse of primary hematologic malignancy remains the leading cause of treatment failure afterallogeneic Hematopoietic cell transplantation (allo-HCT). Multiple studies have shown that presence of measurable residual disease (MRD), at the time of HCT, irrespective of the detection method, is associated with lower overall survival (OS) and relapse-free survival (RFS). In a recent meta-analysis of 11,151 patients with AML, Short et al., reported 5-year disease-free survival (DFS) of 25% for patients who have detectable MRD pre -HCT, compared to 64% for those without MRD. The 5-year OS was 34% and 68% for patients with or without MRD at HCT (JAMA Oncology 2020). In AML patients with genomic evidence of MRD before allo-HCT, myeloablative conditioning (MAC) regimens result in improved survival when compared to reduced intensity conditioning (RIC); (Hourigan et al, JCO 2020). Our center has pioneered and developed Total marrow and lymphoid irradiation (TMLI) that allows for delivery of higher dose of radiation to the sites with high disease burden (bone marrow and lymphoid organs), while sparing healthy tissues. In an ongoing Phase 2 trial (NCT02094794), we delivered TMLI (20Gy) with high dose chemotherapy to patients with high-risk leukemias (ALL or AML) undergoing allo-HCT from an HLA matched or mismatched donor. Here, we report on the results of our prospective analysis on a subset of patients of NCT02094794 who underwent allo-HCT with morphologic remission but had detectable MRD status in their pre HCT bone marrow biopsy (n=30).
Conditioning regimen comprised of TMLI on days -9 to -5 (total of 20Gy), etoposide (60 mg/kg) on day -4, and cyclophosphamide (100mg/kg) on day -2. Unmanipulated peripheral blood stem cells (PBSC) graft from 10/10 HLA matched related or unrelated donor was infused on day 0 and graft-versus-host disease (GVHD) prophylaxis was tacrolimus and sirolimus-based. TMLI was delivered by intensity modulated radiation therapy (IMRT). MRD assay was done by either University of Washington flow cytometry with 0.01% - 0.1 % sensitivity, FISH/conventional cytogenetics, or NGS-based assays.
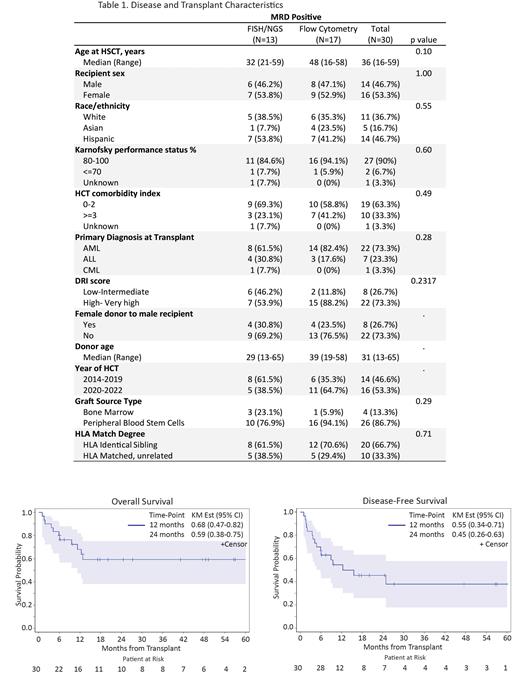
Patient and HCT characteristics are summarized in Table 1. Briefly, the median age at the time of HCT was 36 years (range: 16-59) with the majority of patients receiving PBSC grafts (87%). Donors were match related (67%) or match unrelated (33%). The underlying diagnosis was AML (n=22; 73%), ALL (n=7; 23%), or CML-BC (n=1; 3%). Karnofsky performance status was 80-100 in 90% of patients and HCT-CI ≥3 was seen in 35% of patients. Pre-HCT MRD + status was documented by Flow cytometry MRD assay in 17 patients [AML (n=14) and ALL (n=3)], with mean MRD positivity of 2.9 % (range: 0.02-5.6%) for AML and 0.4% (range: 0.003-1.7%) for ALL patients. Thirteen additional patients had MRD positive status confirmed by FISH/cytogenetics (n=6 patients) or NGS (n=7). With a median duration follow up of 22.3 months (range: 6.0-81.1), the 1 and 2-year OS were 68% (95% CI: 0.47-0.82) and 59% (95% CI: 0.38-0.75), respectively. The 1 and 2- year disease-free survival (DFS) were 55% (95% CI: 0.34-0.71) and 45% (95% CI: 0.26-0.63), while the cumulative incidence (CI) of relapse at 1 and 2 years were 28% (95% CI: 0.13-0.46) and 33% (95% CI: 0.16-0.51), respectively. At day 100 post-HCT, the CI of NRM was 7%, grade 2-4 acute GVHD was 40% and Grade 3-4 acute GVHD was 13%. The CI of NIH moderate- severe chronic GVHD at 1- year was 35% (95% CI: 0.17-0.52). No difference in outcomes were noted in patients who were MRD positive by UWFC vs FISH/NGS testing.
In conclusion, TMLI based conditioning regimen in patients undergoing allo-HCT with MRD+ disease from matched donors is associated with improvement in 2-year RFS/OS and is associated with low NRM compared to historic controls. The current phase 2 study is enrolling patients at our center.
Disclosures
Salhotra:Gilead: Research Funding; Jazz Pharma: Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees; Rigel Pharma: Research Funding; Sanofi: Speakers Bureau; OrcaBio: Research Funding; Kura Oncology: Research Funding; BMS: Research Funding. Al Malki:Tscan: Consultancy. Aribi:Seagen: Consultancy; Kite, a Gilead Company: Consultancy. Aldoss:Amgen: Consultancy, Honoraria; Takeda: Consultancy; Pfizer: Consultancy; KiTE: Consultancy; Jazz: Consultancy; Sobi: Consultancy. Pullarkat:Pfizer: Consultancy, Speakers Bureau; Servier: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau. Becker:Glycomimetics: Research Funding; Accordant Health Services: Membership on an entity's Board of Directors or advisory committees; GPCR Therapeutics: Research Funding; Pfizer: Research Funding. Sandhu:Autolus Therapeutics: Consultancy; City of Hope Medical Center: Current Employment. Ali:Karyopharm: Consultancy; GSK: Consultancy; Pharmaessentia: Consultancy; Blueprints: Speakers Bureau; BMS: Speakers Bureau; Incyte: Research Funding. Koller:NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; treadwell therapuetics: Consultancy, Other: safety review committee; takeda: Consultancy, Speakers Bureau. Marcucci:Ostentus Therapeutics: Current equity holder in private company, Research Funding. Nakamura:Napajen: Consultancy; Sanofi: Consultancy; Blue Bird: Consultancy; International Consortium: Other: consortium chair; Jazz Pharmaceuticals: Consultancy, Other: research collaboration; Miyarisan: Research Funding; NCCN: Other: guideline panel for HCT; Leukemia & Lymphoma Society: Other: grant reviewer; Mt. Sinai: Other: Acute GVHD; Omeros: Consultancy; BMT CTN Steering Committee: Membership on an entity's Board of Directors or advisory committees; NCTN Lymphoma Steering Committee: Membership on an entity's Board of Directors or advisory committees. Stein:Sanofi: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal